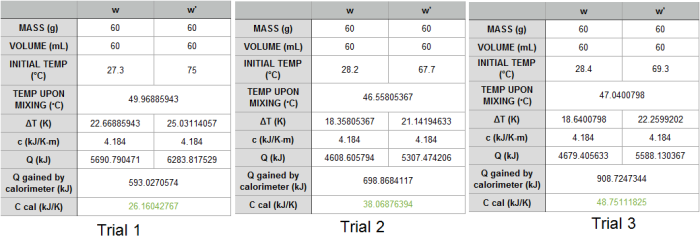
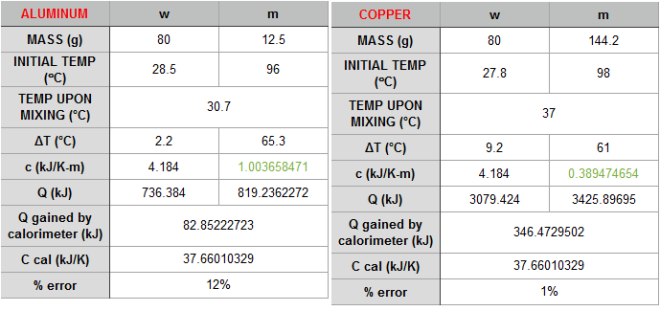
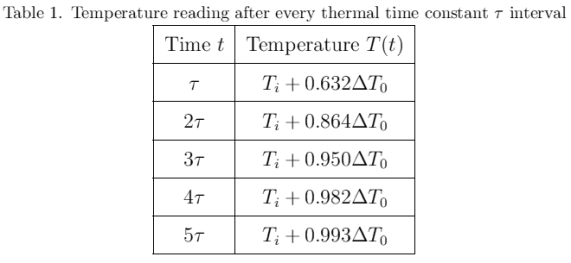
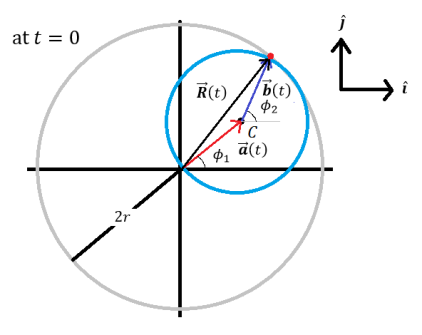
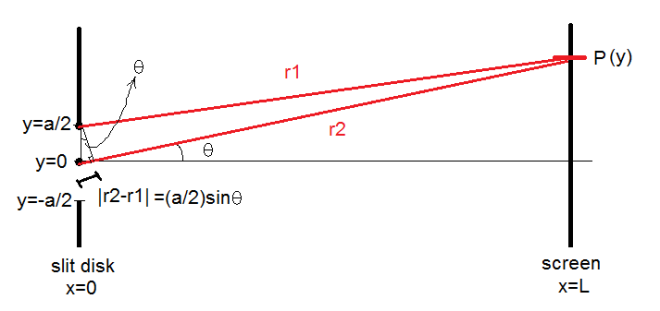
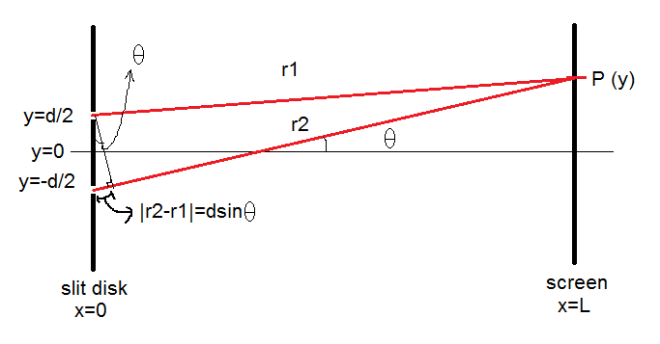
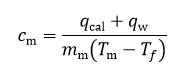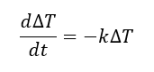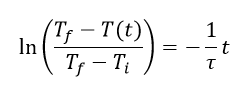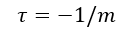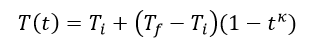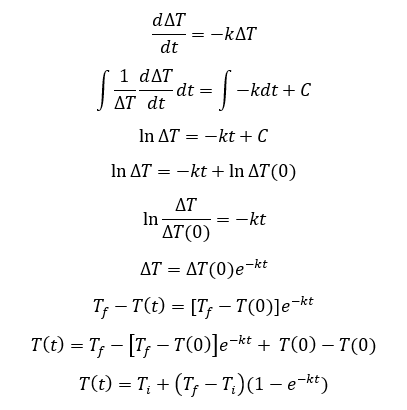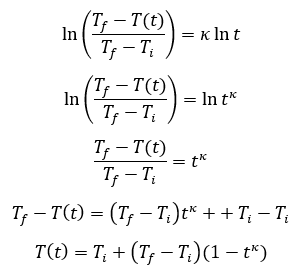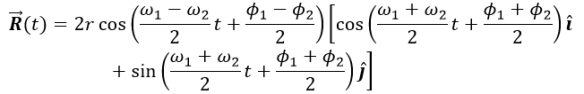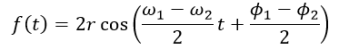The behavior of gases can be modeled by using the concept of an ideal gas. An ideal gas is a gas made up of particles that 1) are infinitesimally small such that the individual particles do not occupy a volume (point particles), 2) only collide elastically, 3) have motions that are independent, i.e., no intermolecular forces manifest between the particles. While such a gas is only a theoretical concept, gases can be approximated to be ideal under certain conditions. For example, by making the container of the gas large, the point particle model of the gas particles becomes more valid.
One can describe the properties of a gas without referring to its microscopic particles. This is through the emergent properties of these particles that can be measured by analyzing the gas as macroscopic entity. Such properties include pressure P, volume V, and temperature T. These properties indicate the state of a gas, and an equation relating these properties is called an equation of state. An equation of state is of great significance because it allows for some of the properties to be calculated instead of measuring it directly. It also implies that an ideal gas cannot have an arbitrary set of values for these properties. That is, for every given ideal gas consisting of N particles, the ordered array Z=(P, V, T) is a unique array of numbers describing its state.
One equation of state is the ideal gas law which assumes an ideal gas
PV=NkT
(1)
where k is the Boltzmann constant with a value of 1.3806488 x 10^-23 m^2•kg•s^-2•K^-1. When certain properties are made constant, one can easily derive the following relationships:
PV=K
(2)
V/T=C
(3)
where K=NkT and C=Nk/P. When temperature is made constant, K is constant and (2) shows the inverse proportionality of pressure and volume. This is known as Boyle’s Law. Meanwhile, under the conditions where the pressure is constant (which is approximately the case in our usual environment), (3) shows that volume is directly proportional to temperature. This is known as Charles’ Law. In this experiments, relations (2) and (3) are verified.
I. Boyle’s Law
A gas carrying-container consisting of two compartments A and B (refer to Figure 1) is subjected to varying pressure by placing different masses on top of the piston in compartment A. Temperature is made constant by immersing compartment B in boiling water where the temperature is unvarying. For each mass 100 g, 150 g, 200 g, 250 g, the pressure and the corresponding volume of compartment A is measured.
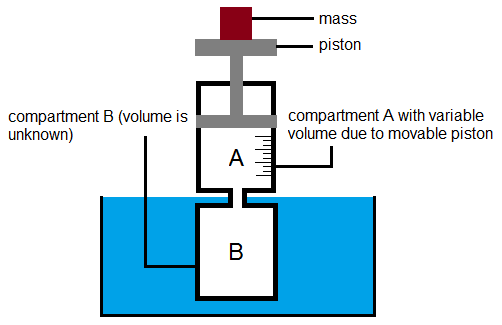
In the actual experiment, compartment B has a much more complicated geometry and thus its volume is unknown. Compartment A is a graduated cylinder and its volume is measurable given its diameter. When the piston is empty, compartments A and B is filled with gas. Noting that the volume of the gas in the container is V=V(A)+V(B) and that V(B) is constant, then from (2)
V=K/P
V(A)+V(B)=K/P
V(A)=K(1/P)-V(B)
(4)
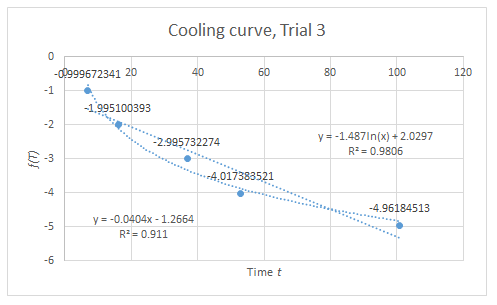
Plotting the volume of A versus the reciprocal of pressure gives a linear curve where the slope is K and the negative of the y-intercept is the volume of B. When the temperature is known, the number of particles N can be calculated since K=NkT.
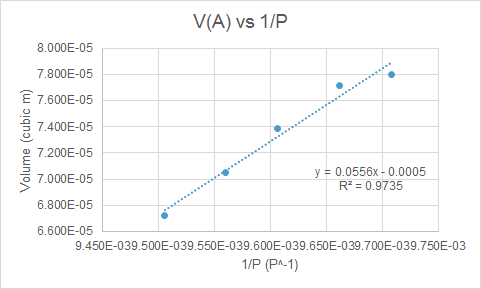
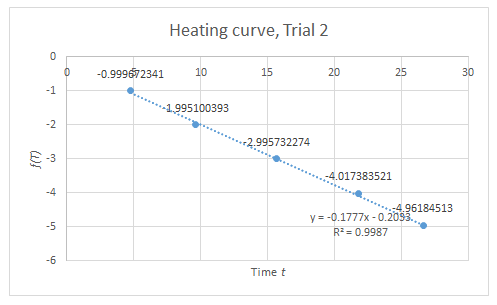
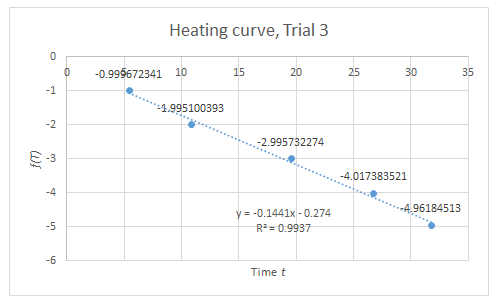
From the data in Figure 2, the calculated volume of B is 500 mL and N=10^19 particles.
II. Charles’ Law
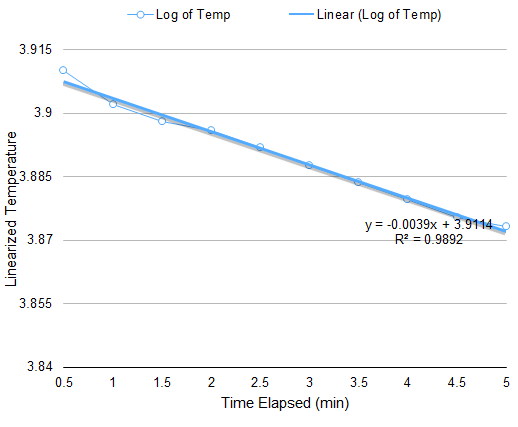
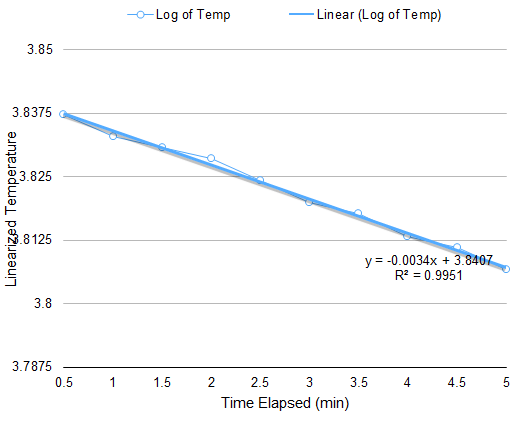
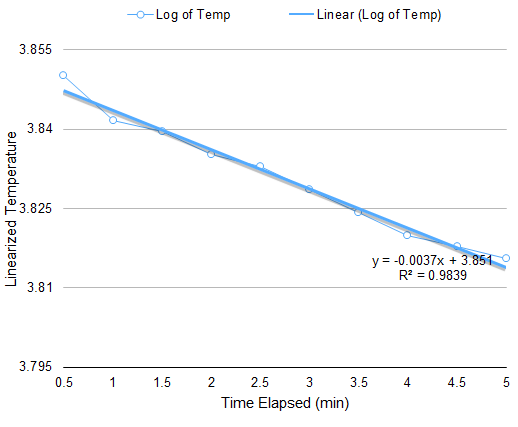
The setup for this part is only a modification of the setup for Boyle’s Law. The apparatus must be subjected to varying temperature. Thus, instead of boiling water, an initially hot volume of water is used, and chunks of ice are added to vary the temperature. At equal intervals of time, the temperature and the corresponding height of the piston are measured. Through a similar approach in the derivation of (4), the relation of volume A and temperature at any given time is
V(A)=CT-V(B)
(5)
Plotting the volume of A versus the temperature gives a linear curve where the slope is C. When the pressure is known, the number of particles N can be calculated since C=Nk/P.
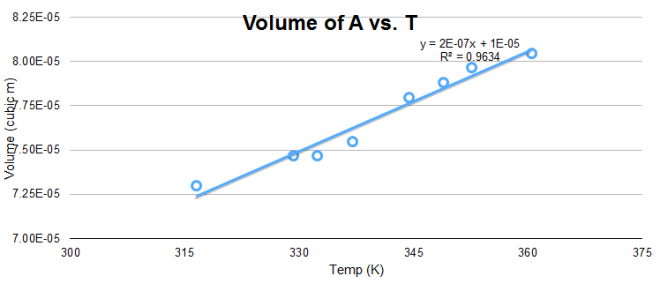
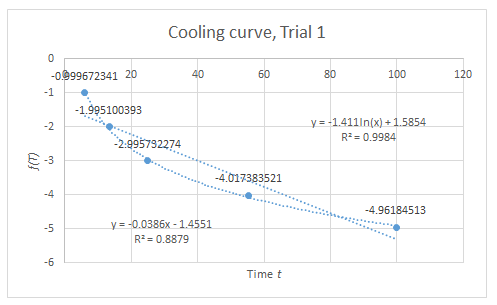
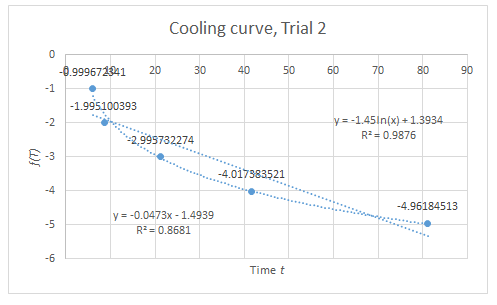
From the data in Figure 3, the calculated N is 10^19 particles, similar to the previous calculation. However, discrepancy arises on the value of the y-intercept since it is expected to be a negative number.
From the R^2 values of the graphs, the trend dictated by (2) and (3) are verified, but only to some extent since these values are not sufficiently close to unity. However, considering the various sources of systematic error, the data suggest that if these error sources were minimized, a more linear trend can be expected.
There are several sources of systematic error for this experiment; the following are only some: First, gas leaks on the container. In the Boyle’s Law setup, when a mass is removed from the piston, it is expected that the piston goes back to its initial height. However, this does not happen. Instead, the piston remains on its position as if the mass is still on top of it. This implies the possibility of gas leak. Second, it takes time for the piston to reach its “equilibrium height” (height where the piston is already static). Thus, it is up to the judgement of the experimenter to determine if the piston is already in steady-state. This source of error has a much significant effect in the Charles’ Law setup. It is assumed in the experiment that at every instant where the temperature is recorded, the piston is already at its corresponding steady-state height. However, if it the piston lags behind too much to reach its steady-state, this assumption becomes invalid. This can account to the much lower R^2 value of the graph in Charles’ Law. Lastly, friction between the piston and the wall of the container can hinder the piston to reach its supposed equilibrium height.